The Clinical Quality Management Plan CQMP establishes the quality management guidelines for tasks related to the NIDCR Protocol. 3 major differences Quality Assurance Quality Control 1 Management system to guarantee integrity of data.
Clinical Laboratory Quality Assurance Plan. 3 Goal is value addition Goal is error prevention 4 Management strategy Error detection methodology 9. Laboratory Quality Assurance QA encompasses a range of activities that enable laboratories to achieve and maintain high levels of accuracy and proficiency despite changes in test methods and the volume of specimens tested. Measurement used to check quality of analytical data. The Clinical Quality Assurance Plan program forms a critical part of the Total Quality Assurance Program of SJANT as it represents the core business patient care.
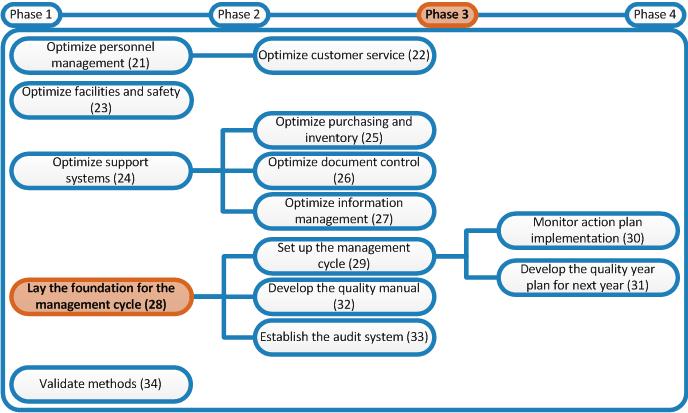 Laboratory Quality Stepwise Implementation Tool From extranet.who.int
Laboratory Quality Stepwise Implementation Tool From extranet.who.int
In hospital-based laboratories the laboratory quality plan may include a section that specifies information that is to be reported to a higher-level authority such as an institution-wide quality committee. The implementation of the Quality Assurance Plan is achieved through a laboratory-wide effort of the entire staff. This may be seen in the organization chart in section 13 and in the. Washington State Office of Laboratory Quality Assurance DEVELOPING A QUALITY ASSURANCE PLAN July 2007 OVERVIEW Each medical test site must establish and follow written policies and procedures for a comprehensive quality assurance QA program. A laboratory QA plan should be responsive to the following items while remaining brief and easy to follow. LOQAM is to document the quality assurance policies and procedures of the EPA Region 4 Laboratory Services Branch LSB laboratory.
A laboratory-wide Quality Assurance Program designed to assess and monitor the ongoing quality of the testing performed in its facilities.
Laboratory Quality Assurance QA encompasses a range of activities that enable laboratories to achieve and maintain high levels of accuracy and proficiency despite changes in test methods and the volume of specimens tested. LOQAM is to document the quality assurance policies and procedures of the EPA Region 4 Laboratory Services Branch LSB laboratory. The Clinical Quality Assurance Plan program forms a critical part of the Total Quality Assurance Program of SJANT as it represents the core business patient care. Laboratory Quality Assurance QA encompasses a range of activities that enable laboratories to achieve and maintain high levels of accuracy and proficiency despite changes in test methods and the volume of specimens tested.
 Source: template.net
Source: template.net
In hospital-based laboratories the laboratory quality plan may include a section that specifies information that is to be reported to a higher-level authority such as an institution-wide quality committee. Include a chart or table showing the laboratory organization and lines of responsibility. The Clinical Quality Management Plan CQMP establishes the quality management guidelines for tasks related to the NIDCR Protocol. 3 Goal is value addition Goal is error prevention 4 Management strategy Error detection methodology. The CLIA regulations Subpart P address specific.
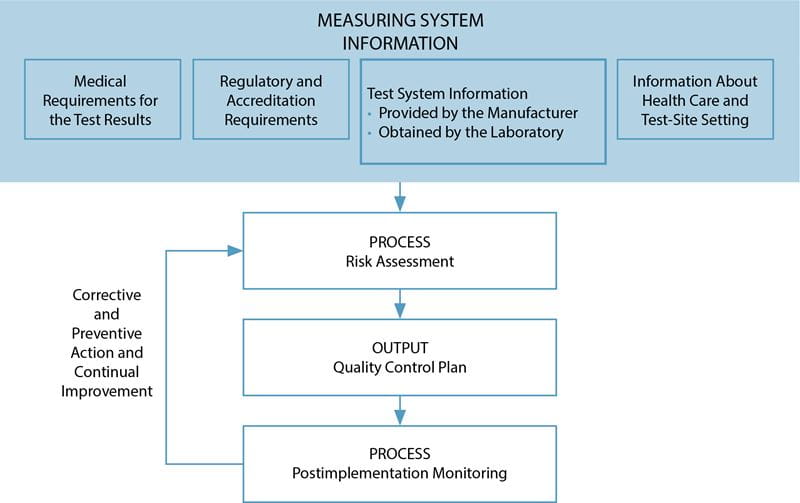 Source: acutecaretesting.org
Source: acutecaretesting.org
Its purpose is to identify and correct problems as they occur and if possible to determine in advance potential problem areas and institute measures for their resolution. Clinical Quality Assurance requires a dynamic process where current clinical performance standards can be set those standards can be distributed and education undertaken. In small stand-alone clinical laboratories the laboratory quality plan usually exists on its own. Minimizing paperwork and improving dependability and quality of data are the intended goals. A laboratory quality assurance plan can present corrective actions whenever the laboratory has been proven to neglect or overlook laboratory quality standards.
 Source: template.net
Source: template.net
You can also check out basic business plans. The CLIA regulations Subpart P address specific. The QA program must be designed to monitor and evaluate the ongoing and overall quality of the total testing process preanalytic analytic. This may be seen in the organization chart in section 13 and in the. Minimizing paperwork and improving dependability and quality of data are the intended goals.
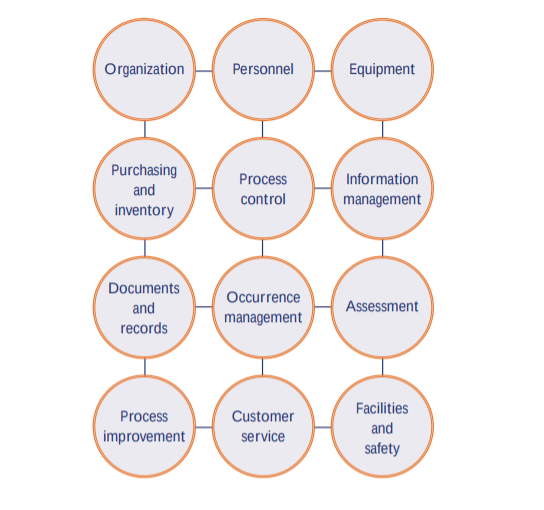 Source: qualio.com
Source: qualio.com
The plan describes a standardized quality control system that maximizes the quality of laboratory. In hospital-based laboratories the laboratory quality plan may include a section that specifies information that is to be reported to a higher-level authority such as an institution-wide quality committee. 3 Goal is value addition Goal is error prevention 4 Management strategy Error detection methodology. This quality management plan provides for continuous monitoring and evaluation of patient care activities within the Department of Pathology and Laboratory Medicine. Clinical Laboratories BCL Quality Assessment Plan is to provide high quality analytical data which is accurate reliable and appropriate for its intended purpose.
 Source: researchgate.net
Source: researchgate.net
In small stand-alone clinical laboratories the laboratory quality plan usually exists on its own. Clinical Laboratories BCL Quality Assessment Plan is to provide high quality analytical data which is accurate reliable and appropriate for its intended purpose. Measurement used to check quality of analytical data. Clinical lab improvement amendments CLIA are a set of regulatory standards for clinical and medical labs that were created to help to make sure medical labs maintain quality assurance. 3 major differences Quality Assurance Quality Control 1 Management system to guarantee integrity of data.
 Source: extranet.who.int
Source: extranet.who.int
Laboratory Quality Assurance QA encompasses a range of activities that enable laboratories to achieve and maintain high levels of accuracy and proficiency despite changes in test methods and the volume of specimens tested. Laboratory organization and responsibility a. The LQM defines quality standards policies and instructions estto ablish a credible laboratory quality assurance program. If a laboratory quality assurance plan is created for a medical industrial or diagnostic laboratory operating in the health science industry this proposed plan is called a laboratory quality assurance plan. The Clinical Quality Management Plan CQMP establishes the quality management guidelines for tasks related to the NIDCR Protocol.
 Source: slideshare.net
Source: slideshare.net
3 Goal is value addition Goal is error prevention 4 Management strategy Error detection methodology 9. In small stand-alone clinical laboratories the laboratory quality plan usually exists on its own. A laboratory-wide Quality Assurance Program designed to assess and monitor the ongoing quality of the testing performed in its facilities. You can also check out basic business plans. It can also help the laboratory be back on track after any instances or circumstances of noncomplianceYou may also see management plans.
 Source: researchgate.net
Source: researchgate.net
3 Goal is value addition Goal is error prevention 4 Management strategy Error detection methodology. Quality assurance vs Quality control Quality Assurance Quality Control 1 Management system to guarantee integrity of data. You can also like software quality assurance plans. Laboratory Quality Assurance QA encompasses a range of activities that enable laboratories to achieve and maintain high levels of accuracy and proficiency despite changes in test methods and the volume of specimens tested. A laboratory-wide Quality Assurance Program designed to assess and monitor the ongoing quality of the testing performed in its facilities.
 Source: template.net
Source: template.net
3 Goal is value addition Goal is error prevention 4 Management strategy Error detection methodology. A laboratory QA plan should be responsive to the following items while remaining brief and easy to follow. If a laboratory quality assurance plan is created for a medical industrial or diagnostic laboratory operating in the health science industry this proposed plan is called a laboratory quality assurance plan. Which the Centers laboratory quality assurance program can operate. The purpose of the CQMP is to identify and document the ongoing processes and activities that will be used to monitor and facilitate quality protocol execution following study initiation.
 Source: slideshare.net
Source: slideshare.net
The purpose of the CQMP is to identify and document the ongoing processes and activities that will be used to monitor and facilitate quality protocol execution following study initiation. The purpose of the CQMP is to identify and document the ongoing processes and activities that will be used to monitor and facilitate quality protocol execution following study initiation. A laboratory quality assurance plan can present corrective actions whenever the laboratory has been proven to neglect or overlook laboratory quality standards. The Quality Assurance Committee. Clinical Laboratories BCL Quality Assessment Plan is to provide high quality analytical data which is accurate reliable and appropriate for its intended purpose.
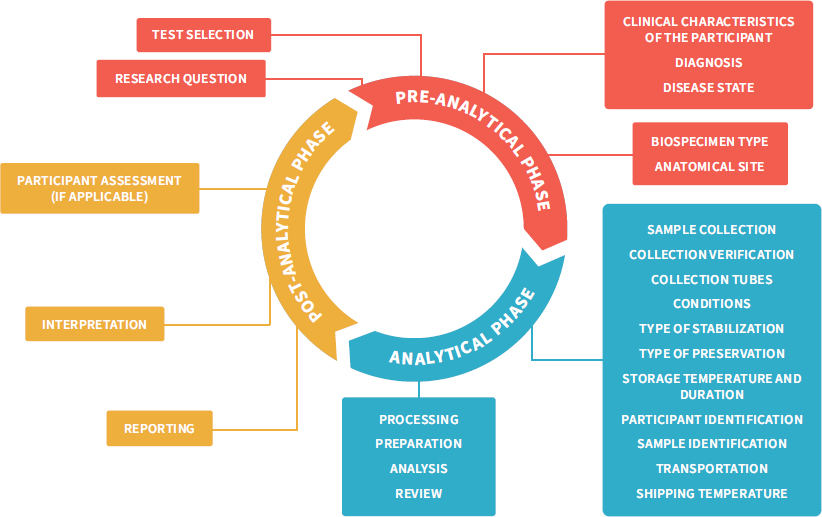 Source: nap.edu
Source: nap.edu
You can also check out basic business plans. A laboratory QA plan should be responsive to the following items while remaining brief and easy to follow. LOQAM is to document the quality assurance policies and procedures of the EPA Region 4 Laboratory Services Branch LSB laboratory. The laboratory organization and personnel are geared toward carrying out the objectives of the Quality Assurance Plan. In small stand-alone clinical laboratories the laboratory quality plan usually exists on its own.
 Source: acutecaretesting.org
Source: acutecaretesting.org
Quality assurance vs Quality control Quality Assurance Quality Control 1 Management system to guarantee integrity of data. The Quality Assessment Plan will enable personnel to establish written procedures to be followed for a comprehensive program of quality assurance as required by the Clinical Laboratory Improvement Amendments CLIA formerly. The implementation of the Quality Assurance Plan is achieved through a laboratory-wide effort of the entire staff. Quality assurance vs Quality control Quality Assurance Quality Control 1 Management system to guarantee integrity of data. The laboratory organization and personnel are geared toward carrying out the objectives of the Quality Assurance Plan.
 Source: researchgate.net
Source: researchgate.net
Clinical lab improvement amendments CLIA are a set of regulatory standards for clinical and medical labs that were created to help to make sure medical labs maintain quality assurance. Which the Centers laboratory quality assurance program can operate. Laboratory Quality Assurance QA encompasses a range of activities that enable laboratories to achieve and maintain high levels of accuracy and proficiency despite changes in test methods and the volume of specimens tested. The Clinical Quality Management Plan CQMP establishes the quality management guidelines for tasks related to the NIDCR Protocol. The Quality Assurance Committee.
 Source: template.net
Source: template.net
Measurement used to check quality of analytical data. 2 Everybodys business Restricted to a specific area and performed by authorized staff. Laboratory Quality Assurance QA encompasses a range of activities that enable laboratories to achieve and maintain high levels of accuracy and proficiency despite changes in test methods and the volume of specimens tested. Measurement used to check quality of analytical data. In hospital-based laboratories the laboratory quality plan may include a section that specifies information that is to be reported to a higher-level authority such as an institution-wide quality committee.
 Source: sciencedirect.com
Source: sciencedirect.com
Measurement used to check quality of analytical data. This may be seen in the organization chart in section 13 and in the. Clinical lab improvement amendments CLIA are a set of regulatory standards for clinical and medical labs that were created to help to make sure medical labs maintain quality assurance. 3 Goal is value addition Goal is error prevention 4 Management strategy Error detection methodology 9. Which the Centers laboratory quality assurance program can operate.
 Source: researchgate.net
Source: researchgate.net
Clinical Laboratories BCL Quality Assessment Plan is to provide high quality analytical data which is accurate reliable and appropriate for its intended purpose. A defined system of quality assurance practices and operational policies a quality system is essential for ensuring that data generated from analytical processes are well-defined and defensible. LOQAM is to document the quality assurance policies and procedures of the EPA Region 4 Laboratory Services Branch LSB laboratory. The purpose of the CQMP is to identify and document the ongoing processes and activities that will be used to monitor and facilitate quality protocol execution following study initiation. A laboratory quality assurance plan can present corrective actions whenever the laboratory has been proven to neglect or overlook laboratory quality standards.
 Source: conductscience.com
Source: conductscience.com
The QA program must be designed to monitor and evaluate the ongoing and overall quality of the total testing process preanalytic analytic. 3 Goal is value addition Goal is error prevention 4 Management strategy Error detection methodology 9. The CLIA regulations Subpart P address specific. Minimizing paperwork and improving dependability and quality of data are the intended goals. 3 Goal is value addition Goal is error prevention 4 Management strategy Error detection methodology.
 Source: slideplayer.com
Source: slideplayer.com
The implementation of the Quality Assurance Plan is achieved through a laboratory-wide effort of the entire staff. You can also like software quality assurance plans. Laboratory organization and responsibility a. A laboratory quality assurance plan can present corrective actions whenever the laboratory has been proven to neglect or overlook laboratory quality standards. Minimizing paperwork and improving dependability and quality of data are the intended goals.





