This 2-day course provides attendees with an understanding of safety assurance cases and how they can be applied to combination products to demonstrate safety and facilitate pre-market review communications with the FDA. This part of ISOIEC 15026 specifies minimum requirements for the structure and contents of an assurance case.
Fda Assurance Case. The National Medical Device Curriculum is a set of four fictitious case studies intended to help academic institutions and medtech companies understand FDAs medical device regulatory processes. The maintenance of the valid state must be ensured over the entire life cycle of the system. The common issues and resolutions related to assurance cases. This technical information report provides guidance on how to complete an Assurance Case Report in order to comply with the new additional FDA pre-market requirements for infusion pumps.

The assurance case is a method for reasoning about systems appropriate for scientists and engineers. FDA launches four case studies to explain medical device regulation. The FDA believes that better understanding of regulatory processes will accelerate the delivery of innovative medical devices to. CSA vs CSV. The National Medical Device Curriculum is a set of four fictitious case studies intended to help academic institutions and medtech companies understand FDAs medical device regulatory processes. Wwwfdagov Case for Quality 6 Why Risk to patients from quality issues and hampered innovation in manufacturing and product development practices High industry focus on.
The assurance case is a method for reasoning about systems appropriate for scientists and engineers.
Warning Letter issued for the second site. The FDA recognizes the value of using advanced technologies to enable the industry to make medicines of more reliable quality. That a specified set of. The FDA believes that better understanding of regulatory processes will accelerate the delivery of innovative medical devices to.
 Source: ispe.org
Source: ispe.org
The assurance case is a method for reasoning about systems appropriate for scientists and engineers. The FDA recognizes the value of using advanced technologies to enable the industry to make medicines of more reliable quality. Wwwfdagov Non-Product CSV Modification Impact Activity Current Approach Modified Approach. The infrastructure used must be qualified. The assurance case is a method for reasoning about systems appropriate for scientists and engineers.
 Source: researchgate.net
Source: researchgate.net
The FDA initiated this pilot to gather information about industry concerns with safety assurance cases and how the FDA can provide more clarity on the development of safety assurance cases. The assurance case is a method for reasoning about systems appropriate for scientists and engineers. FDA CSA Computer Software Assurance The Case for Quality. Most of the combination products are drug delivery devices like infusion pumps and would be expected to have information like safety assurance cases be included in. CSA vs CSV.
 Source: perfval.com
Source: perfval.com
That a specified set of. Safety Assurance Case and FDA. That provides a convincing and valid. Medical device safety assurance case guidance. A safety case is a structured argument supported by a body of evidence that provides a compelling comprehensible and valid case that a system is safe for a given application in a given environment.
 Source: researchgate.net
Source: researchgate.net
FDA launches four case studies to explain medical device regulation. It represents the wise choice of many alternatives William A. The FDA initiated this pilot to gather information about industry concerns with safety assurance cases and how the FDA can provide more clarity on the development of safety assurance cases. What Happened FDA placed both sites under import alert within two weeks of the second inspection. In the regulated environment all computer-based systems used must be validated before they are put into productive use.
 Source: perfval.com
Source: perfval.com
Wwwfdagov Non-Product CSV Modification Impact Activity Current Approach Modified Approach. The infrastructure used must be qualified. Assurance Case An Excellent Tool for Overcoming the Challenge. Guidance development centered around assurance activities for this category. This part of ISOIEC 15026 specifies minimum requirements for the structure and contents of an assurance case.
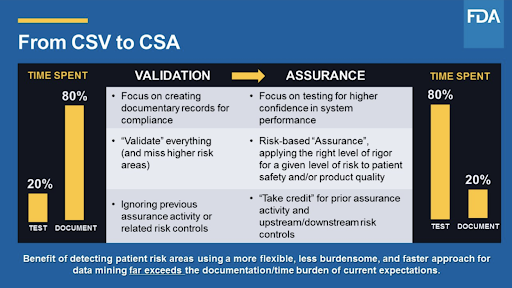 Source: aodocs.com
Source: aodocs.com
An assurance case includes a top-level claim for a property of a system or product or set of claims systematic argumentation regarding this claim and the evidence and explicit assumptions that. This 2-day course provides attendees with an understanding of safety assurance cases and how they can be applied to combination products to demonstrate safety and facilitate pre-market review communications with the FDA. Warning Letter issued for the second site. Most of the combination products are drug delivery devices like infusion pumps and would be expected to have information like safety assurance cases be included in. FDA CSA Computer Software Assurance The Case for Quality.

What Happened FDA placed both sites under import alert within two weeks of the second inspection. The FDA requires that a safety assurance case be included in 510 k submissions for infusion pumps. It represents the wise choice of many alternatives William A. The National Medical Device Curriculum is a set of four fictitious case studies intended to help academic institutions and medtech companies understand FDAs medical device regulatory processes. An assurance case addressing safety is called a safety case.
 Source: ispe.org
Source: ispe.org
The FDA recognizes the value of using advanced technologies to enable the industry to make medicines of more reliable quality. Wwwfdagov Non-Product CSV Modification Impact Activity Current Approach Modified Approach. The FDA requires that a safety assurance case be included in 510 k submissions for infusion pumps. That a specified set of. It represents the wise choice of many alternatives William A.

FDA launches four case studies to explain medical device regulation. The FDA recognizes the value of using advanced technologies to enable the industry to make medicines of more reliable quality. It represents the wise choice of many alternatives William A. FDA launches four case studies to explain medical device regulation. The National Medical Device Curriculum is a set of four fictitious case studies intended to help academic institutions and medtech companies understand FDAs medical device regulatory processes.

This technical information report provides guidance on how to complete an Assurance Case Report in order to comply with the new additional FDA pre-market requirements for infusion pumps. This 2-day course provides attendees with an understanding of safety assurance cases and how they can be applied to combination products to demonstrate safety and facilitate pre-market review communications with the FDA. Assurance Case An Excellent Tool for Overcoming the Challenge. FDAs New Guidance for Software Assurance Quality is never an accident. The FDA requires that a safety assurance case be included in 510 k submissions for infusion pumps.
 Source: researchgate.net
Source: researchgate.net
The common issues and resolutions related to assurance cases. Safety Assurance Case and FDA. What Happened FDA placed both sites under import alert within two weeks of the second inspection. Regulations exist all over the world for the protection and safety of consumers. FDA Assurance Cases 21 22 Feb 2008 Based on Open Group Paris 23 April 2007 slight revisions of Open Group San Diego 31 January 2007 major rewrite of HCSS Aviation Safety Workshop Alexandria Oct 56 2006 Based on University of Illinois ITI Distinguished Lecture Wednesday 5 April 2006 based on ITCES invited talk Tuesday 4 April 2006.

The National Medical Device Curriculum is a set of four fictitious case studies intended to help academic institutions and medtech companies understand FDAs medical device regulatory processes. The National Medical Device Curriculum is a set of four fictitious case studies intended to help academic institutions and medtech companies understand FDAs medical device regulatory processes. Regulations exist all over the world for the protection and safety of consumers. An assurance case addressing safety is called a safety case. Medical device safety assurance case guidance.
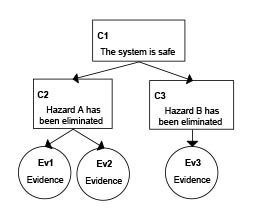 Source: insights.sei.cmu.edu
Source: insights.sei.cmu.edu
The FDA believes that better understanding of regulatory processes will accelerate the delivery of innovative medical devices to. Wwwfdagov Case for Quality 6 Why Risk to patients from quality issues and hampered innovation in manufacturing and product development practices High industry focus on. Understand how safety assurance case can help to address limitations with common risk management methods practices Ask FDA questions about risk management and safety assurance cases FDA participants are available for questions after the session content has been presented. That provides a convincing and valid. FDA Assurance Cases 21 22 Feb 2008 Based on Open Group Paris 23 April 2007 slight revisions of Open Group San Diego 31 January 2007 major rewrite of HCSS Aviation Safety Workshop Alexandria Oct 56 2006 Based on University of Illinois ITI Distinguished Lecture Wednesday 5 April 2006 based on ITCES invited talk Tuesday 4 April 2006.
 Source: researchgate.net
Source: researchgate.net
That a specified set of. In the regulated environment all computer-based systems used must be validated before they are put into productive use. An assurance case includes a top-level claim for a property of a system or product or set of claims systematic argumentation regarding this claim and the evidence and explicit assumptions that. It is always the result of high intention sincere effort intelligent direction and skillful execution. And the structure templates and approach for using assurance cases to achieve three important goals.
 Source: johner-institute.com
Source: johner-institute.com
CSA vs CSV. The FDA requires that a safety assurance case be included in 510 k submissions for infusion pumps. FDA CSA Computer Software Assurance The Case for Quality. That a specified set of. This 2-day course provides attendees with an understanding of safety assurance cases and how they can be applied to combination products to demonstrate safety and facilitate pre-market review communications with the FDA.
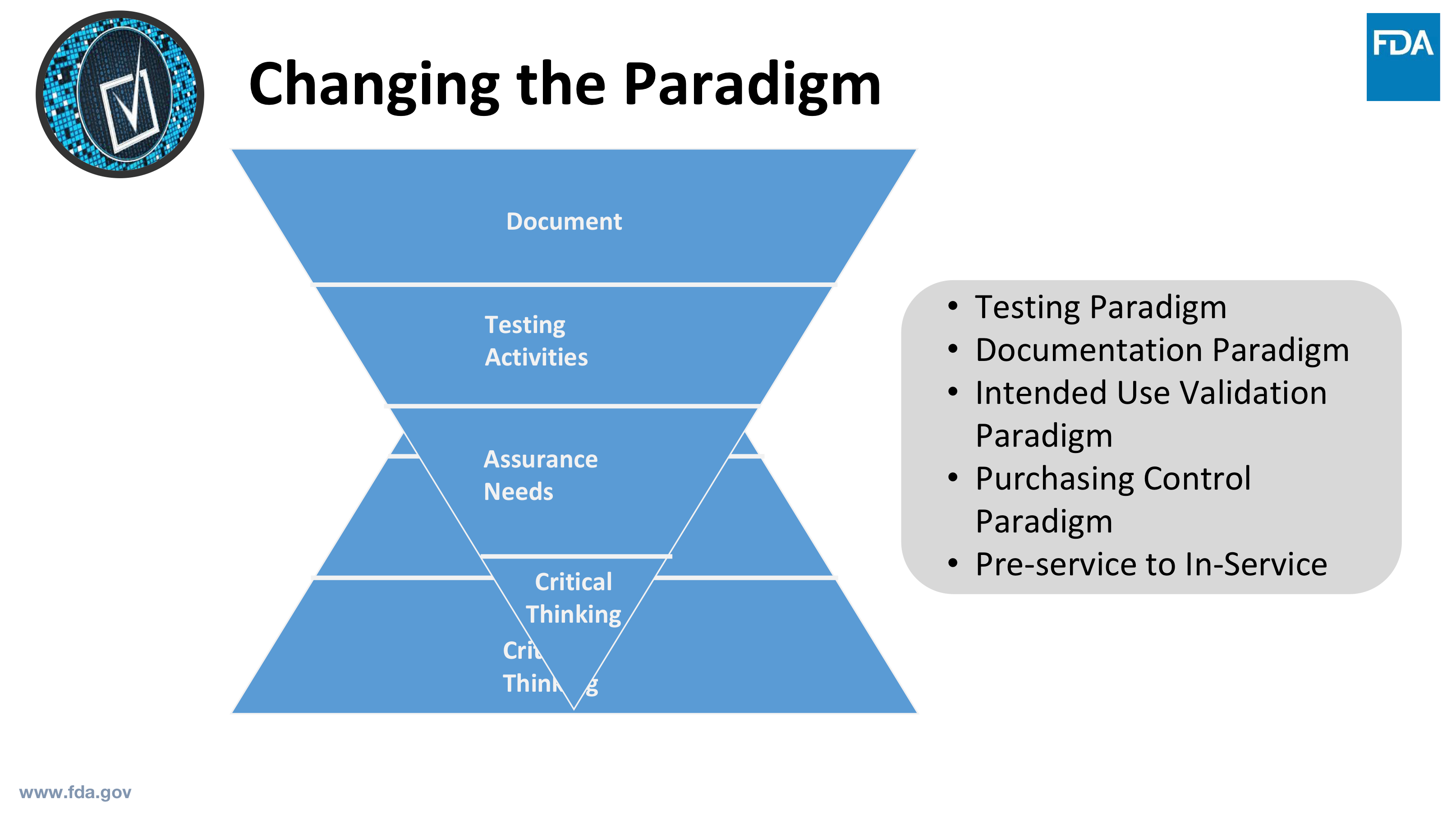 Source: greenlight.guru
Source: greenlight.guru
The study revealed an inclination towards medical device software which is the product software not taking into. Contact FDA Follow FDA on Facebook Follow FDA on Twitter View FDA videos on YouTube Subscribe to FDA RSS feeds FDA Homepage Contact Number 1-888-INFO-FDA 1-888-463-6332. Regulations exist all over the world for the protection and safety of consumers. Safety Assurance Case and FDA. It represents the wise choice of many alternatives William A.

Most of the combination products are drug delivery devices like infusion pumps and would be expected to have information like safety assurance cases be included in. This technical information report provides guidance on how to complete an Assurance Case Report in order to comply with the new additional FDA pre-market requirements for infusion pumps. FDA Assurance Cases 21 22 Feb 2008 Based on Open Group Paris 23 April 2007 slight revisions of Open Group San Diego 31 January 2007 major rewrite of HCSS Aviation Safety Workshop Alexandria Oct 56 2006 Based on University of Illinois ITI Distinguished Lecture Wednesday 5 April 2006 based on ITCES invited talk Tuesday 4 April 2006. What Happened FDA placed both sites under import alert within two weeks of the second inspection. The study revealed an inclination towards medical device software which is the product software not taking into.
 Source: gessnet.com
Source: gessnet.com
The common issues and resolutions related to assurance cases. This technical information report provides guidance on how to complete an Assurance Case Report in order to comply with the new additional FDA pre-market requirements for infusion pumps. The FDA requires that a safety assurance case be included in 510 k submissions for infusion pumps. Safety Assurance Case and FDA. A documented body of.





